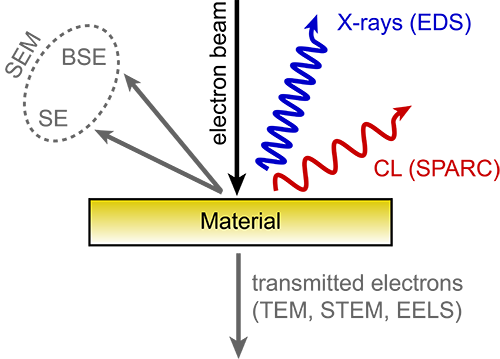
Cathodoluminescence emission can be used to explore many fundamental properties of matter. It can be used to study light transport, scattering, electronic structure of a material, resonant phenomena and much more. It thus presents a valuable source of information for fundamental research as well as applied research with a direct link to industry. Different types of cathodoluminescence detection, also known as imaging modes, can open up new insights and layers of information about your sample. Here are the six most commonly used imaging modes.
Fast-intensity imaging
To obtain cathodoluminescence contrast intensity imaging is commonly performed. A fast photomultiplier tube (PMT) detector is used for large-scale imaging, enabling rapid inspection of large areas, and efficient region-of-interest finding. A filter wheel is present for spectral differentiation.
Applications: This mode is particularly useful for imaging larger areas which is often required in geological applications for example.
Read more in the technical note: Cathodoluminescence intensity mapping.

Hyperspectral imaging
Visualizing the wavelength distribution (spectrum) of the material in a parallel manner
Applications: This imaging technique can help you to obtain valuable information on the local optical and structural properties of (nano)materials, such as semiconductors, as well as geological samples.
Read more in the technical note: Hyperspectral cathodoluminescence imaging.

Angle-resolved imaging
Studying how your sample emits and scatters light is possible with angle-resolved cathodoluminescence. Each point of the acquired camera image corresponds to a unique emission angle: this allows characterization of material performance in terms of directivity.
Applications: Angular profiles acquired with this imaging mode are very valuable in the field of nanophotonics.
Read more in the technical note: Angle-resolved cathodoluminescence imaging.

Polarimetry and polarization filtered spectroscopy
Measuring the polarization of light reveals in what direction the electromagnetic fields oscillate. This technique allows measuring the polarization state (Stokes vector) of cathodoluminescence for different emission angles.
Application: This mode can be used for comprehensive measurements of coherence, scattering, and chirality.
Read more in the technical note: Polarization-filtered cathodoluminescence imaging.

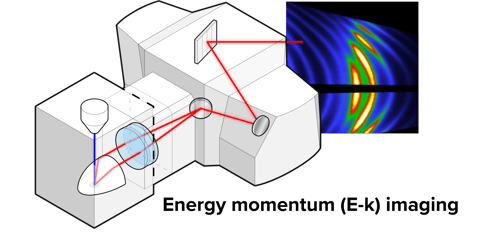
Energy-momentum (E-k) Imaging
This imaging mode allows users to acquire high-resolution datasets resolved both in angle and wavelength, for any given location on the sample. It is a great tool for tracking the directionality through energy and momentum space with very high precision.
Applications: E-k can be applied to a wide range of dispersive and anisotropic (photonic) systems, paving the way for a broad range of studies in such applications as solid-state lighting, photovoltaics, and sensing.
Read more in the technical note: Energy-Momentum cathodoluminescence imaging.
Time-resolved cathodoluminescence imaging
Time-resolved cathodoluminescence is a technique in which you look at the time dynamics of the cathodoluminescence emission process. Performing time-resolved imaging is possible with the optional Lab Cube time-resolved module or streak camera. The Lab Cube can be used to measure lifetimes as well as the second-order autocorrelation function of the emission, also known as g(2)
Applications: Time-resolved cathodoluminescence imaging is highly relevant for a wide range of applications, including semiconductors for photovoltaics, light-emitting devices, as well as for (single) emitters for quantum information processing and sensing.
Read more in the technical notes: Lifetime cathodoluminescence mapping and Cathodoluminescence g(2) imaging.

.png)





.gif?width=227&name=CL%20FAQ_Animated%20Banner%20(1).gif)