CLEM microscopy is the perfect tool for studying the complex relation between form and function in biology.
CLEM enables you to benefit from the labeling power of FM and the high resolution imaging power of EM. Using CLEM microscopy means you can collect both functional information given by the FM image and structural information given by EM. Moreover, the region of interest can be selected based on FM and subsequently imaged at high resolution using EM.
While FM and EM are powerful techniques in their own right, they are not without drawbacks. Fluorescence imaging provides valuable functional information but is very limited in resolution, whereas EM, although high resolution, lacks functional information, meaning that identifying cells based on EM alone is error-prone. Neither imaging mode by itself can therefore provide complete information. Performing CLEM allows you to have the best of both techniques.
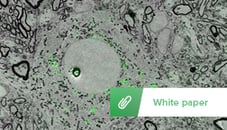
Figure 1: Image of projection neurons in songbird brain under a correlative light and electron microscope. Imaging was performed using the SECOM platform (DELMIC) mounted on a Thermo Fisher Quanta 250 FEG SEM.
Fluorescence microscopy
Fluorescence microscopy owes its popularity to the immense variety of labelling strategies that can be employed. Samples can be dyed, immuno-labeled, or tagged using genetically encoded fluorescent proteins. Furthermore, thanks to the spectral properties of these fluorescent tags, multiple labels can be identified simultaneously using excitation light of different wavelengths. In this manner, different parts of the cell, and therefore different cellular functions, can be imaged simultaneously, forming a multi-color fluorescence image.
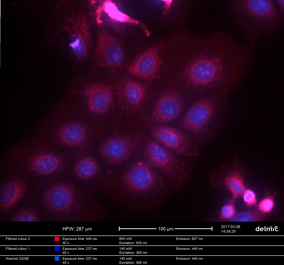
Figure2: Multi-color FM image of HeLa cells, transfected with Lifeact-mcherry (actin) and counterstained with DAPI (nuclei). Samples courtesy N. Liv, UMC Utrecht
Fluorescence is the phenomenon of the emission of light upon the absorption of a photon. As shown in the diagram, when a molecule in ground state absorbs a photon, it is excited to a higher energy state from where it can decay to intermediate lower energy states. What decay channels are available depends on the molecule and its environment. The difference in energy between the ground state and the intermediate state may be given out as a photon, the emission of which is called fluorescence. Fluorescence microscopy is based on the imaging of fluorescent molecules that are selectively attached to different parts of a cell or organism.
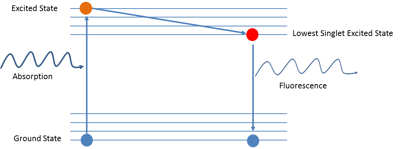
Figure 3: Example of a Jablonski diagram of a fluorophore. This diagram depicts the processes that occur which cause an object to convert light from one wavelength to fluorescent light. Ground state energy states are shown in blue. Highest energy state is shown in orange and the lowest single excited state is shown in red.
Electron microscopy
Electron microscopy is a powerful method for acquiring high resolution structural information at the nanometer scale. Because the wavelength of accelerated electrons is much shorter than that of visible light, the diffraction barrier of light can be overcome and much smaller features can be visualized.
What distinguishes light from electron microscopy is not only the resolution. The type of contrast that is typically measured in EM is very distinct from FM. Whereas in FM one can only detect specific macromolecules that have been labeled, in EM one acquires primarily contextual information. In other words, you see everything there is. Examples of useful applications of EM in the life sciences include membrane structures such as the endoplasmic reticulum, the Golgi apparatus, and vesicular structures.
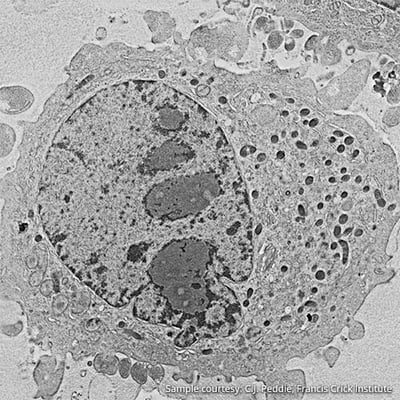
Figure 4: Scanning electron image of HeLa cell.
Electron microscopy, often performed in a Scanning Electron Microscope (SEM), focuses a beam of high energy electrons (typically upto 30 keV) onto the surface of a sample. A host of scattering processes takes place upon the interaction of the beam with the atoms in the material, resulting in the generation of, among others, low energy secondary electrons (SE’s) and high energy backscattered electrons (BSE’s). These are detected with dedicated detectors. By scanning the beam over the sample, an image is made, which contains different kinds of information about the sample depending on the electrons detected, such as topography and density.

.png)