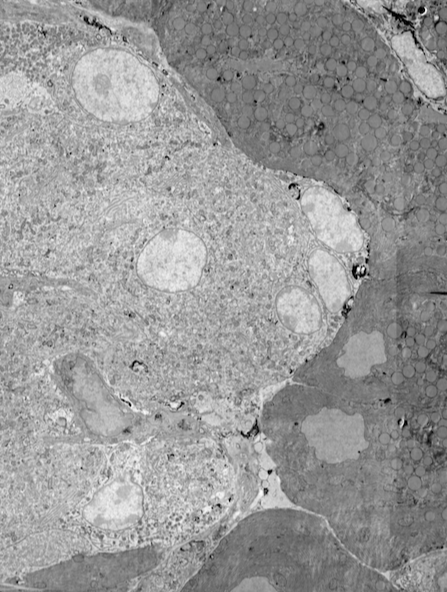
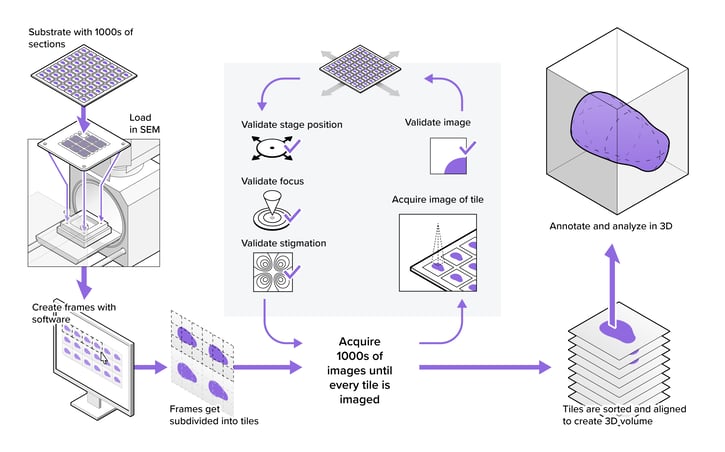
In life sciences, electron microscopy (EM) helps biologists to examine cells, cellular processes, and organelle architecture at nanometer-range resolution. With more than 90 years of development, electron microscopy is now a mature, well-established technique applied in many fields. Recent improvements in automation and stability have enabled EM to produce data for increasingly large biological projects, where large numbers of samples or large volumes are imaged and analyzed. Still, most EM workflows have issues that limit their power as a biological investigation tool.
Most of the time, the development of a scientific technique is primarily focused on pushing the physical parameters achieved with the technique, for example, spatial or time resolution or speed. Sooner or later, however, also other aspects start playing an important role, such as time- and cost-efficiency, throughput, and ease of use. In the case of traditional electron microscopy workflows, the low throughput and the requirement for a high level of human intervention are the most limiting factors.
Due to the limited throughput of EM, microscope operators often are forced to strike a compromise between the size or number of samples, the resolution used for imaging, and the required imaging time. In addition, operators are often required to supervise the system to ensure consistent imaging quality throughout the project. By removing the bottleneck of throughput, the power of EM as a biological investigation tool increases drastically, since larger or more samples can be imaged automatically, without operator intervention.
Read more: optimizing for high sustained throughput in large-scale electron microscopy
.png)