Choose the right product for your research
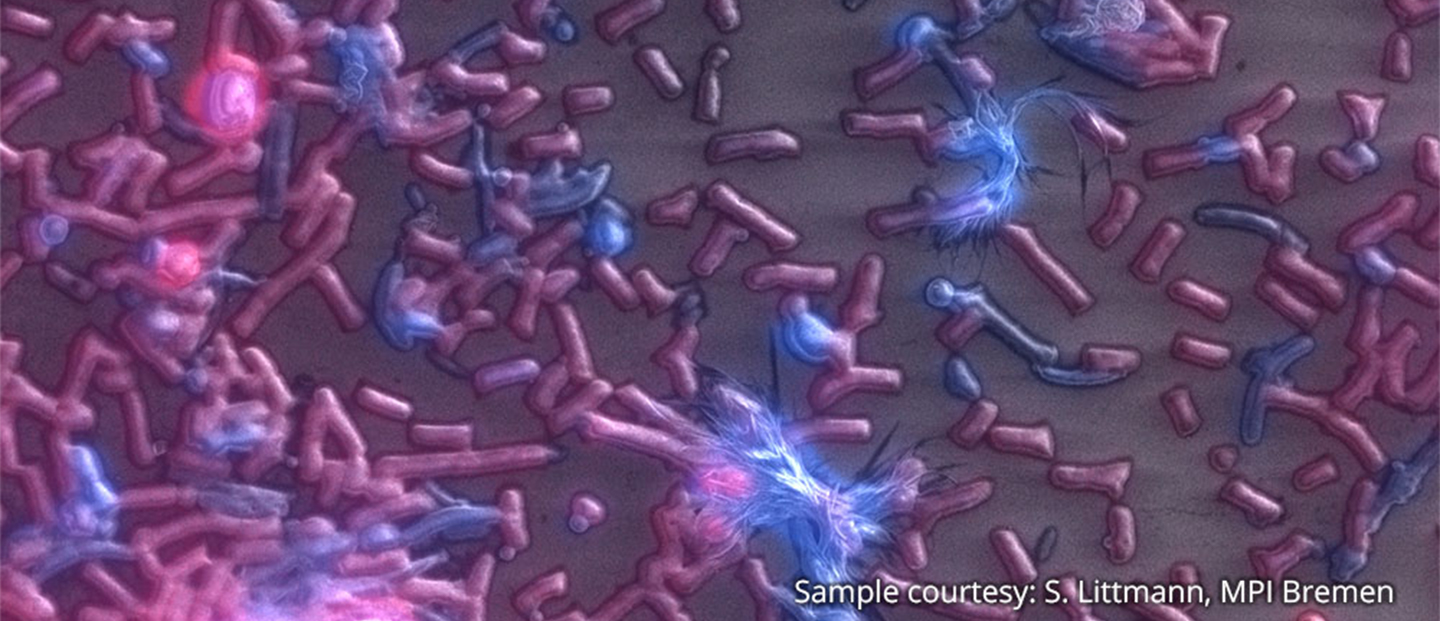
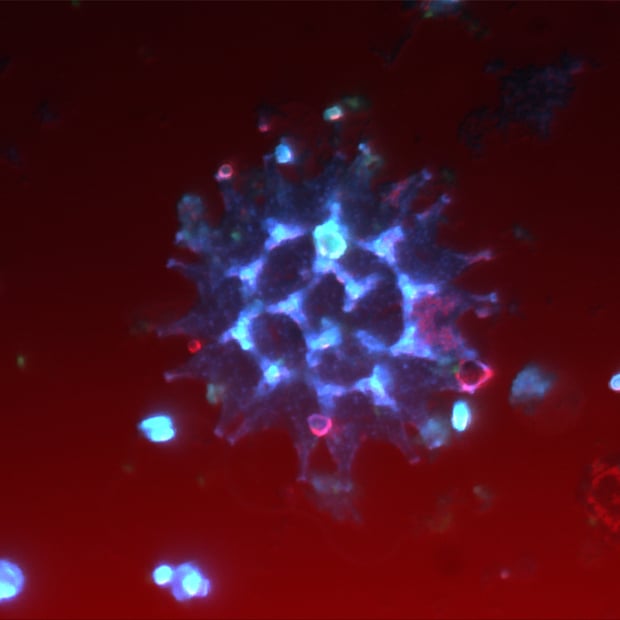
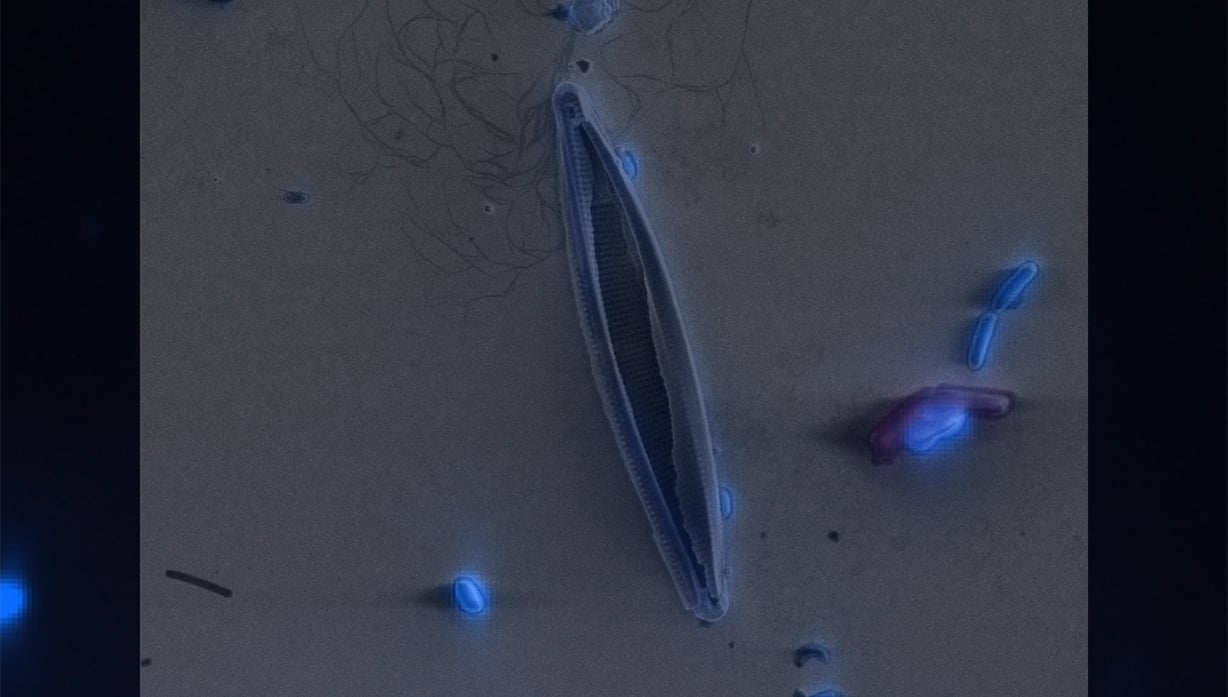
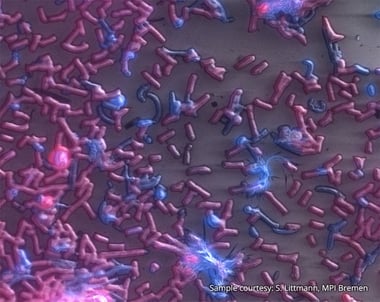
Delmic CLEM solutions consist of powerful integrated correlative light and electron microscopy systems, which can help you understand more about your microbiological sample.
Don’t know which product fitting your research?
Talk to an expert